Main Benefits
Why Choose Humankine cGMP Recombinant Proteins?
Animal Component Free
Expressed in stable human HEK293 systems with active human conformation and post-translational modifications.
Lot-to-lot Consistency
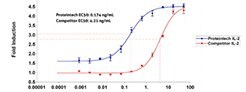
Proteintech's in-house GMP manufacturing process ensures high lot-to-lot consistency in identity, purity, and bioactivity.
ISO13485 Certified
Rigorous ISO13485 certified and cGMP compliant quality processes and extensive documentation, thorough QC testing and traceability.
Scalability
Robust scalability from pre-clinical to clinical and commercial quantities.
Additional Information
Applicable Solutions

Seamless Preclinical to Clinical Transition
The master cell line and manufacturing process are the same for GMP and RUO cytokines, minimizing risk when scaling up and transitioning to the clinic.
View All ProductsYou might also be interested in
Frequently Asked Questions
How are HumanKine GMP cytokines different from the RUO cytokines?
GMP proteins come with extensive documentation for traceability, as well as additional quality control testing and quality assurance reviews, whereas the RUO grade line offers reliable products which are more cost-effective during early research and development. As the manufacturing process is the same for RUO and GMP products, HumanKine offers a seamless preclinical-to-clinical transition of product lines, saving a significant amount of time and money.
What is the stability of HumanKine proteins?
Unless specified otherwise in the product information sheet, our products are formulated to ensure the stability of lyophilized proteins at room temperature. It is advised to store lyophilized products at -20°C to -80°C. For reconstituted solutions of most products, short-term storage at 4°C is recommended. Extended storage of the protein solution should be at -20°C to -80°C. Keep in mind that each freeze/thaw cycle may lead to some protein denaturation, so it is not recommended to subject aliquots to multiple freeze/thaw cycles.
Is HumanKine GMP product manufacturing certified by the FDA or other regulatory agencies?
The US-FDA does not audit or certify manufacturing facilities that produce ancillary materials. HumanKine GMP products are manufactured under the ISO13485 quality management system adhering to USP and European pharmacopeia recommendations to ensure potency, purity, and safety. The manufacturing facility can be audited by the end user upon request.